What Is a Clinical Trial Management System?
A clinical trial management system (CTMS) is software specifically designed for the unique needs of clinical trials. This software has modules for project management, as well as management for all processes across a clinical trial, starting with protocol development and concluding with clinical study reporting and study closeout.
Clinical trial management software’s features are what set it apart from other types of systems. CTM systems perform more than just one function (such as handling patient records or performing recruitment calls). In fact, they perform a host of managerial services and business processes that far surpass an electronic data capture (EDC) system or an interactive voice response (IVR) system on its own. The evolution of CTMS has allowed a wide range of professionals to eliminate multiple systems and implement more cohesive processes and trials. In short, these systems save considerable time and money.
Industries that use clinical trial management solutions — including pharmaceuticals, biotechnology, clinical research, academic medical centers, and cancer centers — should expect a minimum of the following management capabilities:
- Project, Program, and Site: Oversight and monitoring of activities, high-level project and program goals, and sites
- Trial and Site Specific: Investigator and site recruitment and metrics for each site and trial
- Subject: Subject enrollment, records, visits, reports, and status
- Study: Adherence to protocol concerning data collection, documentation, task tracking, and central data access
- Relationships: With investigators, institutional review boards (IRBs), and ethics committees
- Finances: Cost tracking and management of grants, payments, reimbursements, claims, and financial disclosures
- Supplies: Tracking and ordering
- Reporting: Regarding study data, dashboards, and operational reports
How Clinical Trial Management Systems Can Help with Clinical Trial Financial Management
A clinical trial is a financially complex project. With continuously changing budgets that parallel the changes within the trials themselves, accurate and consistent management can be quite challenging. A CTMS can help track costs, billing, invoices, and grants at a central, granular level.
At this detailed level, study executives can better monitor and control the cost of a trial. By producing reports, they can also use the software to find possible inefficiencies and savings in an overall program, as well as in specific trial projects. Here are other financial reports that a CTMS can produce:
- Cost Recovery: Detail any recovered costs from the method and from budget changes.
- Patient Enrollment: Specify the patient enrollment patterns and methods that are successful for future trials.
- Business Prospects: Calculate the ROI for new business prospects, taking into account current business information, patterns, and trends.
- Staff Utilization: Use schedules and appointments to calculate staff workloads and capacity so that trial managers can adjust if necessary.
A CTMS should also support the functions of project management, compliance, and recruitment. Project management in clinical trials includes predictable tasks such as planning, milestone tracking, and workflows. It also includes more clinical trial-specific tasks, such as trial and site planning and investigator management. Learn how to put all the project management tools and theory to use in this project management guide.
Compliance functions in a CTMS include billing, research, reporting and analytics, and data verification. Recruitment functions in a CTMS include patient information databases, integration with web and social media recruitment sites, text messaging, and participant payments.
What to Look for in a Clinical Trial Management System
A solid CTMS makes clinical trial administration and management easier and saves projects and organizations money. Good systems manage data in a logical, user-friendly way and ensure security and centralization. Streamlined processes and the transparency of activities and finances increase trial outcomes.
If a business has a solid grasp of its capacities and goals, it can choose a system wisely. When selecting an appropriate CTMS for your business, start by making the following decisions:
- Decide which managerial personnel should be involved in the CTMS adoption process.
- Determine the budget for the system.
- Figure out which parts of your current system work well and which could use improvement.
- Pinpoint which organizational goals will be relevant to the CTMS.
- Determine what actionable data the system needs to produce.
- Find out if the software needs to work with existing systems.
- Figure out how much time you can allot for setup and installation.
- Determine the type of training and the amount of software support your company will need.
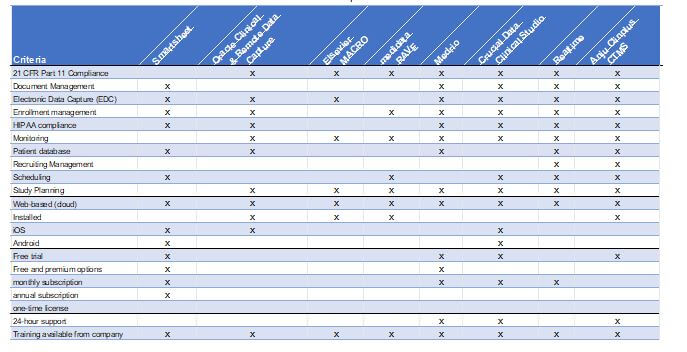
If you are ready to compare clinical trial management software, look at the features of each program and determine which ones have the software you need. Also, be skeptical of newer, untested platforms. Here are some of the features that you should consider:
- Regulatory compliance
- Integration with current software
- Quick adoption
- Document management
- Electronic data capture
- Free trial
- Subscription type (annual, monthly, one-time license)
- Number of users capable
- Platform (web-based or installed)
- Mobile enabled (iOS or Android)
- Open source
- Site communication
- Supply chain management
- Patient scheduling and communication
Use this free chart to compare the top ten CTMSs and their features.
What Is a Clinical Trial Management Process?
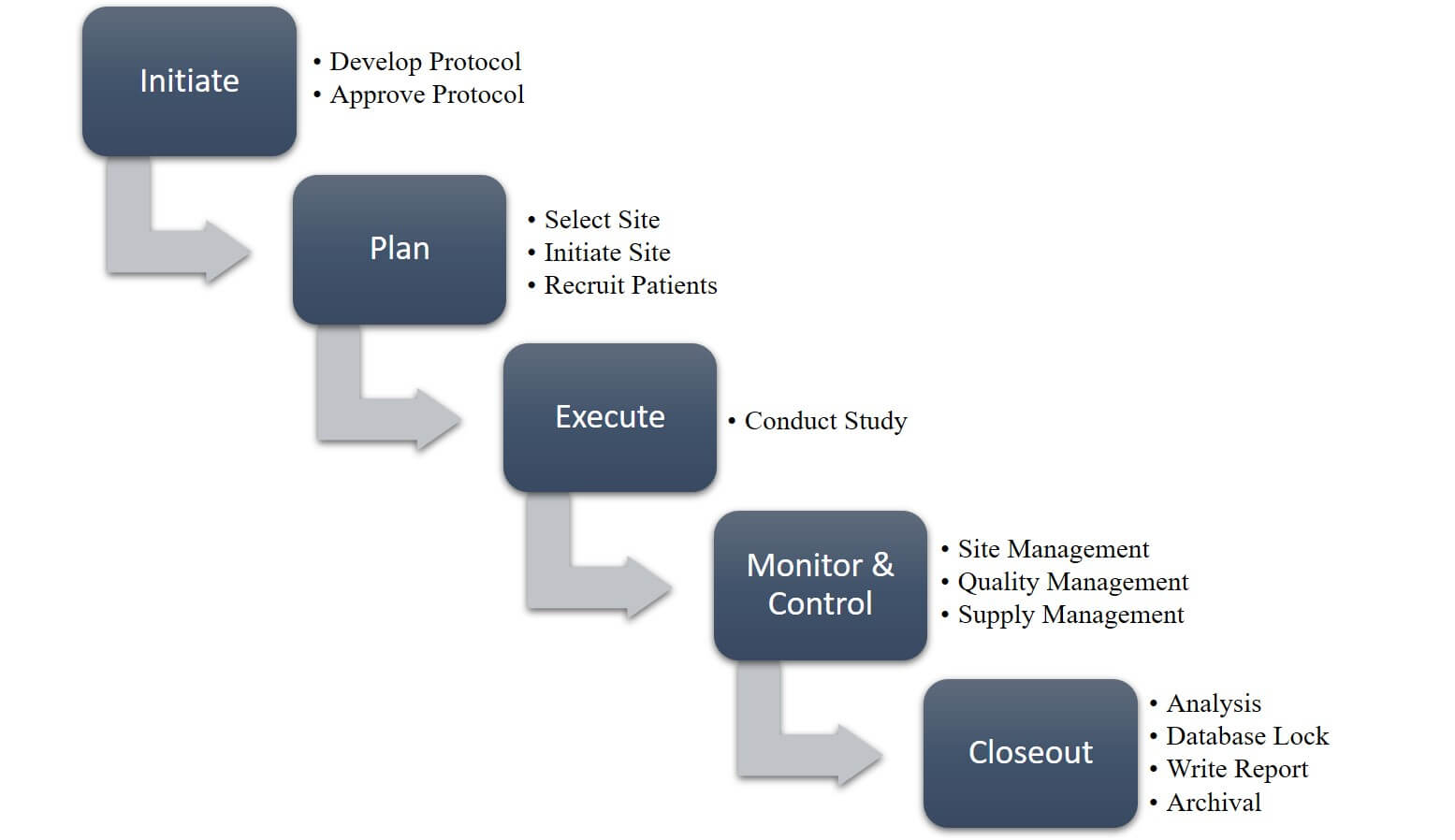
All clinical trials require the same type of coordinated systems and processes, regardless of their size and scope. The design of a trial constitutes merely one element of clinical trial management. It is the effectiveness of a clinical trial’s management plan and processes that make a trial successful.
An overview of clinical management processes takes into account all the steps necessary to get from initiation to closeout. Executives should begin the clinical data management process by keeping the conclusion in mind in order to ensure that the end deliverable is in constant view. Maintaining this vision will enable you to run statistically sound analyses on your clinical trial database by keeping it valid and error-free. Data managers should design a database based on study protocol to make sure that it can support a study’s data items and collection frequency.
Clinical Trial Management Process Overview
Melissa Peda, Clinical Data Manager at the Fred Hutch Cancer Research Center, discusses performing clinical trials from a business management perspective. “Successful trial managers recognize that they need to include traditional business functions, such as marketing, sales, and client relationship management,” she says.
Trial recruitment is especially dependent on good process management, as trials can easily fail because of participant attrition. Below are some features that can attract and keep participants:
-
Evidence-based trials
-
Academic group participation
-
Extra recruitment staff
-
Personal interest on the part of patients
-
Easy-to-understand trial explanations
Another important part of the clinical trial management plan is how staff members publish and distribute their findings. This part of the plan should not be an afterthought, but rather part of the original plan. It should include everyone who made a substantial contribution to the study. It’s crucial to publish results, regardless of a trial’s level of success. Some researchers consider the failure to report results to be scientific misconduct.
Globally, there is no required level of clinical trial project management training, education, or experience. However, with so many regulations, rules, laws, and best practices for trials themselves, a good clinical manager displays the knowledge and maturity to respect and incorporate these parameters. The UK’s National Institute for Health Research (NIHR) offers managers a clinical trials toolkit regarding clinical research guidelines. The U.S. does not furnish a similar, singular document that governs the entire country, but individual institutions that fund or host their own clinical research offer their own versions.
Two additional areas of management are supply management in clinical trials and site management in clinical trials.
Clinical trial supply management is the practice of ensuring that all clinical trial sites have the materials they need to run efficiently. Such materials include not just the office supplies, but also the pharmaceuticals that are integral to an investigation. This management of pharmaceuticals (i.e., investigational product management) can complicate clinical trial supply management considerably. For example, investigational product management in clinical trials can extend to multiple sites worldwide that are beholden to their country’s regulations. The responsibility of a site to maintain its nation’s regulations can make management complex and difficult. In addition, supply management also entails the management of the value chain of drug reconciliation, return, and destruction.
Clinical trial site management is the comprehensive management of a site and can involve several studies with different arms or simply one large study. Sometimes, the study project manager performs this function with the intent of ensuring that there is one consistent point of contact throughout the study.
Quality Management Plan in Clinical Trials
The clinical trial quality management plan (QMP) includes all the activities that verify that a trial is safe and meets its requirements. This plan reviews work processes and project deliverables. Quality managers develop the QMP to correspond with the protocol and the sponsor’s requirements.
Writing a quality management plan in clinical trials starts with the study protocol and the sponsor’s strategic plan to maintain consistency. Additionally, the sponsor may already have a data management plan template for clinical trials to follow. Because staff members use this plan to help deliver the required level of quality, it is crucial to consider its contents carefully. Here are some things to consider when writing a plan:
- Make Standards Clear: When writing a quality management plan, the staff should write all standards, including the clinical trial data standards, clearly. Aspirational standards are fine, but well-researched, realistic standards are more important. In the case of QMPs, the sponsor’s or principal investigator’s experience can help determine what is realistic.
- Welcome Suggestions: Discuss with your staff any ideas they have for innovative solutions. In order to generate the best outcomes, your staff needs to understand the purpose of the plan while it is still in draft form.
- Clarify Roles and Responsibilities: To increase buy-in among the staff, clearly articulate the expected roles and responsibilities. All staff members have some level of quality requirement, based on their position.
Use this sample quality management plan template to help you write a quality management plan. The template provides instructions and space to develop your clinical quality management schedule and activities, and is designed in a professional format that you can easily share with stakeholders.
Download Quality Management Plan Template
Word | PDF | Smartsheet
Managing Clinical Trials Successfully
As mentioned, managing clinical trials is a complex enterprise. Managers should ensure that they incorporate strong clinical practice guidelines, the ethics of nonmaleficence, respect for persons, beneficence, and privacy guidelines. Regardless of the country in which your researchers work, the same standards of ethics and scientific integrity should apply.
For a clinical trial to be successful, no matter what phase or trial type, the manager must use a structured approach. Each phase of a trial should include a clear objective of what the trial needs to accomplish. To learn more about the specific phases of clinical trials, read “Understanding the Phases of Clinical Trials.”
Establishing the protocol is the first thing to accomplish before the start of any trial, as it will provide guidance for the entire trial. Any changes that investigators make to the protocol should be considered amendments that must go through the appropriate regulatory agency.
Use this free research protocol template to develop a research protocol, which will serve as the first major milestone in developing a clinical trial. With its simple, guiding instructions, this template will help you think through and develop all aspects of the clinical trial in a professional, easy-to use format.
Download Research Protocol Template — Word
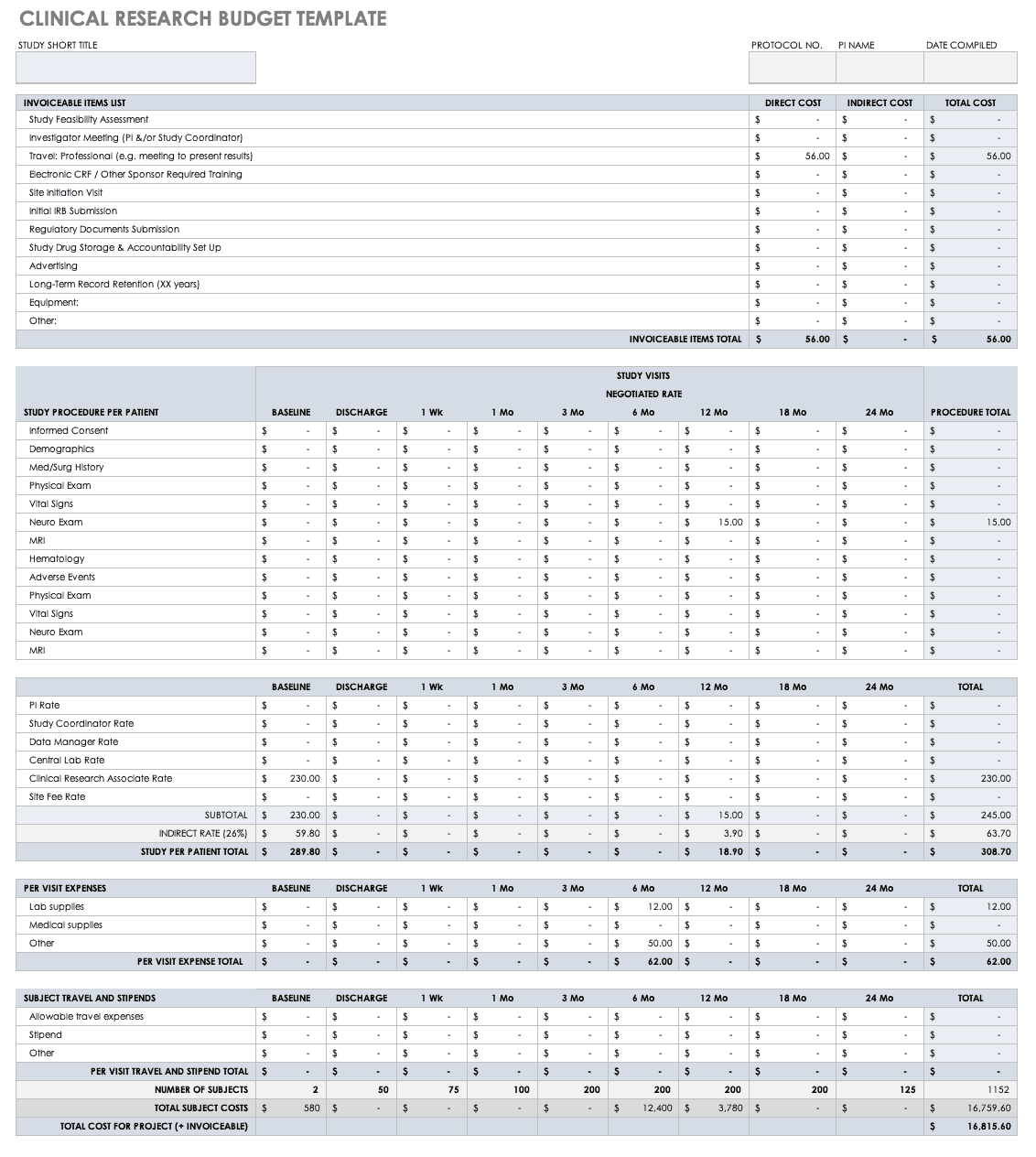
The next critical piece of clinical research is budget development, in which the investigators identify the costs for all necessary activities, assign the financial responsibilities of each partner, and figure out the process necessary to recover costs throughout the study.
Use this free clinical research budget plan template to start thinking through and developing a research budget. The template gives you an example budget to follow, with examples of direct, indirect, and total costs for expenses related to a clinical trial. The inlaid formulas and negotiated rates by study visit also give you an example how to professionally present, tally, and document your costs.
Download Clinical Research Budget Plan Template — Excel
Finally, before starting any clinical research, you must consider its feasibility. Create a feasibility checklist based on the quality of the protocol. The checklist should consider the experience of the sponsor or clinical research organization, as well as how realistic it is to get the right population to participate. Three factors — the quality of the protocol, the experience of the sponsor or organization, and the probability of getting the right population — point to either a viable study or one that needs more planning.
A structured approach also includes looking for risks and mitigating them in the planning. For example, the staff could mitigate any barriers to participation by ensuring that the research question is an important one and by guaranteeing that the protocol and the method the team is using to collect its data make sense to possible participants. Other factors that contribute to a successful trial include the following:
- Setting a time scale
- Designing a careful recruitment process
- Keeping the demand for patients and clinicians low
- Defining the necessary resources in advance
- Detailing the tasks and the standard for their completion
- Retaining a dedicated research staff
Researchers must apply the same depth of thought to the actual project management plan that they apply to the clinical trial itself. This consideration includes the development of background research, the methodology, the subject guidelines, and the drug chemistry. A well-designed clinical trial is essential, but research cannot deliver results without a well-designed project plan. Hence, the project manager must create and employ systems and techniques that are helpful and responsive to the needs of the trial and its people.
Clinical Data Managers Are Project Managers Too
Clinical data managers often have backgrounds in project management and work as project managers in clinical studies. Their role can include leading the planning of the study, coordinating the study, and ensuring the study’s completion through the assessment and monitoring of the project’s staff.
Peda explains best practices for project management in clinical trials: “You need to use project management tools to map out when you should deliver project pieces. You need to be able to backtrack and develop a timeline, while working with key personnel’s schedules. These project management skills are critical to clinical trials because you need to constantly facilitate the work of others and know everyone’s work status for the project.” Two of the tools that Peda uses in her lead role of coordinating, planning, and completing studies include Gantt charts and Kanban boards. Try these free Excel Gantt chart templates, and learn all about Kanban boards here.
Here are some of Peda’s additional best practices for clinical project management professionals:
- Figure out who the stakeholders are and address them in a communication plan.
- Develop a process that completes several activities at once during the start-up phase.
- Partnering the data management plan with risk-based monitoring activities.
- Continually ask whether the study is prioritizing patient safety and data integrity.
- Debrief with the staff after every study regarding lessons learned.
Clinical research requires comprehensive management. Sometimes, staff roles overlap or staff members take on multiple roles. For example, a principal investigator can also act as a study’s statistician, or a data manager can also act as a programmer or internal auditor.
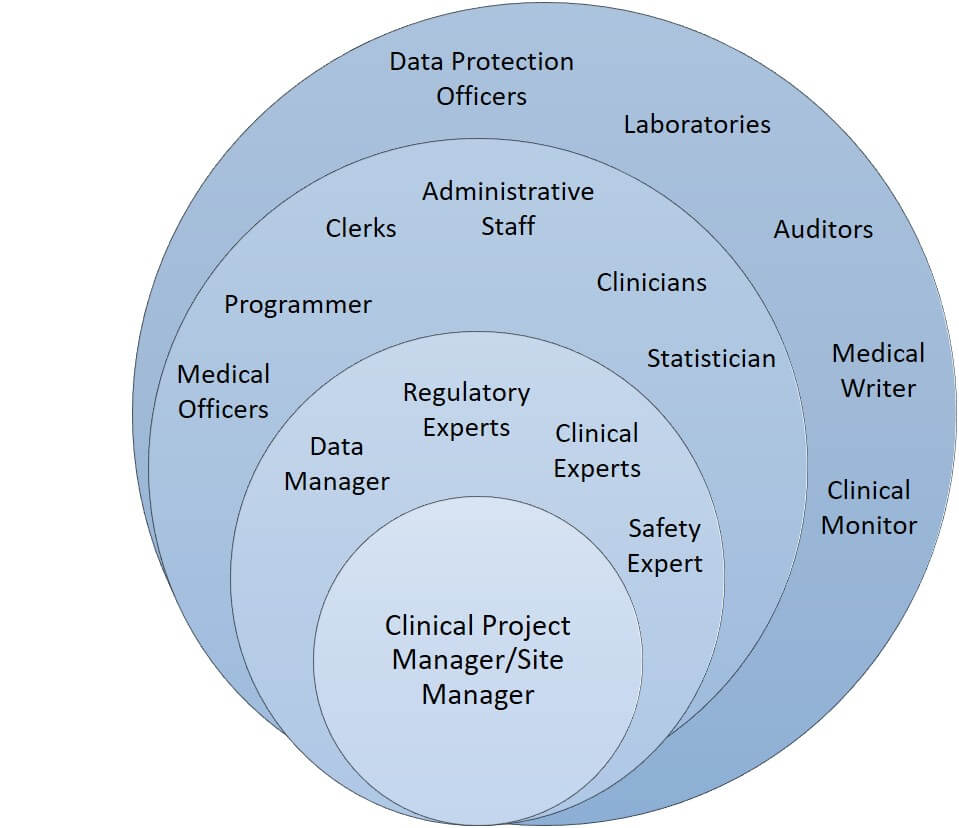
Clinical Trial Management and Staff Roles
The trial management lead role ensures that a project is supported by strong collaboration, communication, and leadership. The lead could also be responsible for clinical trial contract management and account management. The graphic above shows one way to organize the staff involved in a trial: The innermost circle contains the management lead; the next circle includes the expert staff members involved in developing the study; the next circle includes the active staff members on the study; the outermost circle contains the external staff members.
Clinical trials include many positions, and there are numerous ways to organize a trial’s staff, especially considering that some trials use contract research organizations (CROs) as the source for external skills.
Improve Clinical Trial Management Software and Processes with Smartsheet for Healthcare
Empower your people to go above and beyond with a flexible platform designed to match the needs of your team — and adapt as those needs change.
The Smartsheet platform makes it easy to plan, capture, manage, and report on work from anywhere, helping your team be more effective and get more done. Report on key metrics and get real-time visibility into work as it happens with roll-up reports, dashboards, and automated workflows built to keep your team connected and informed.
When teams have clarity into the work getting done, there’s no telling how much more they can accomplish in the same amount of time. Try Smartsheet for free, today.
Any articles, templates, or information provided by Smartsheet on the website are for reference only. While we strive to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability, or availability with respect to the website or the information, articles, templates, or related graphics contained on the website. Any reliance you place on such information is therefore strictly at your own risk.
These templates are provided as samples only. These templates are in no way meant as legal or compliance advice. Users of these templates must determine what information is necessary and needed to accomplish their objectives.