Life Sciences
Accelerate life-saving innovation with intelligent work
Manage complex product lifecycles to deliver treatments and devices to patients faster.
30%
boost in program productivity
Roche Diagnostics reduced time spent on administrative tasks and streamlined implementations for a better patient experience.
300
hours saved per year on document reviews
Galenicum streamlines communication, consolidates data and processes to save time.
50%
reduction in medical equipment repair time
Jeisys Medical significantly improves operational efficiency.
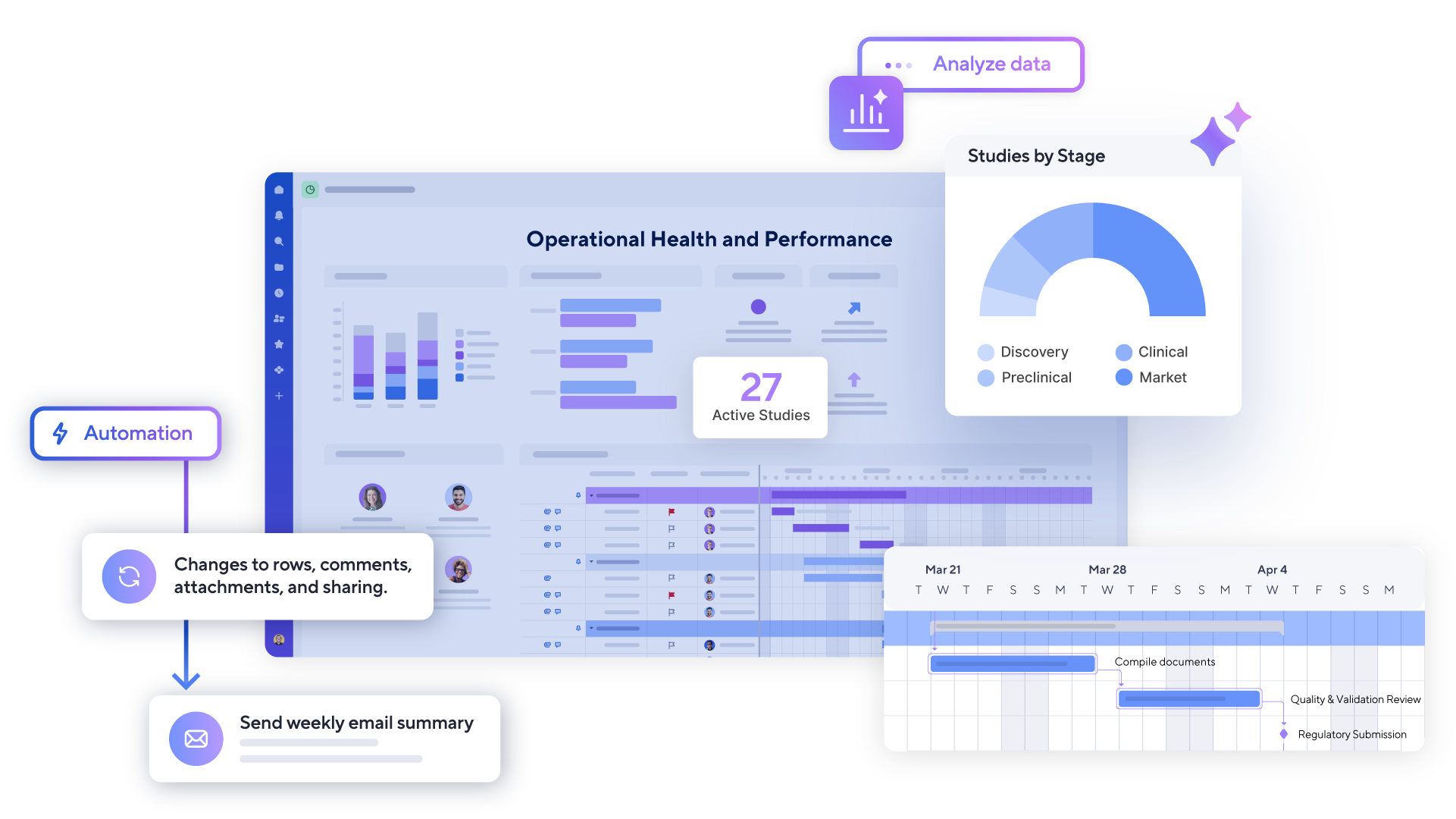
Unify teams, fuel growth, and drive results that scale
From discovery to launch, Smartsheet is the trusted intelligent work management platform that empowers teams across your organization to drive operational efficiency, optimize resources, and accelerate time to market.

Solutions you need to accelerate execution
and drive innovation in life sciences
FAQ
Enterprise-grade security and compliance
Smartsheet is built with enterprise-grade security to protect your data and maintain compliance with global standards. Our platform features robust encryption (both in transit and at rest), role-based access controls, and detailed audit logs to safeguard your information. Smartsheet adheres to leading security and compliance frameworks including SOC 2, ISO 27001, GDPR, and FedRAMP. Smartsheet can also be used to receive, maintain, or transmit certain types of Protected Health Information (PHI) in accordance with your HIPAA obligations. For specific product eligibility, control configurations, and recommendations, please see our HIPAA article. For more details, visit our Trust Center.
Audit trails and data integrity
Smartsheet provides core capabilities that support traceability, including activity log and cell history for a real-time audit trail of changes made; automated workflows that streamline approvals and notifications, tracking who approved what and when, which helps with adherence to SOPs; and forms that allow for error-free data collection to protect data integrity.
Smartsheet integrates seamlessly with a wide range of enterprise systems, including popular tools for CRM, ERP, ITSM, and collaboration like Salesforce, Jira, Microsoft 365, Google Workspace, Tableau, Docusign. Additionally, Smartsheet can integrate with eClinical systems including CTMS via Data Shuttle or our extensible API. Learn more about integrations on our Integrations page.
Yes. Smartsheet unites people, data, and AI in one place to eliminate execution silos and facilitate seamless, secure cross-functional collaboration.
At Smartsheet, we prioritize data protection and privacy. Our AI is designed with security and transparency in mind so that you can enjoy its benefits safely. Additionally, the data you submit to our AI tools is not used to train our LLM providers’ models. Learn more in our AI whitepaper.
Ready to get started?
continue exploring